Effect of low Electro-Magnetic-Frequency Therapy (EMF) / Rife on Circulating Tumour Cell (CTC) count in cancer patients
The study is being conducted at the National Institute of Integrative Medicine (NIIM), A/Prof Dr Karin Ried (Director of Research, NIIM), Prof Avni Sali (Director, NIIM) and Joy Chu (Research Nurse NIIM), in collaboration with Dr Peter Eng at the Eng Medical Centre, Melbourne, and Dr Taufiq BinJemain at the Willow Vale Clinic at the Gold Coast. The study has ethics approval.

Project explanation and objectives
Electro-Magnetic-Frequency Therapy (EMF), developed by Royal Raymond Rife in the early 1900’s, uses low frequency waves (<25×106 Hz) – lower than those emitted by mobile phones (1×108 Hz) – for the treatment of cancer and/or pathogens.
A small number of published studies (Ried 2023, Zimmerman 2013, Le Chapellier 2014, Barbault 2009), and our own pilot study (Ried 2018-2024 unpublished) provided promising results with a reduction in Circulating Tumour Cells (CTC) and overall improved results, including partial remission, in cancer patients after EMF therapy. Patients suffering chronic fatigue due to viral reactivation also showed improvement.
We will use Circulating Tumour Cell (CTC) blood testing – a technique we established at the NIIM lab in 2014, having done more than 4000 CTC tests to date – to assess EMF treatment effectiveness. The CTC count is directly related to cancer status, with lower CTC counts being associated with reduced cancer risk. More info at: http://niim.com.au/ctc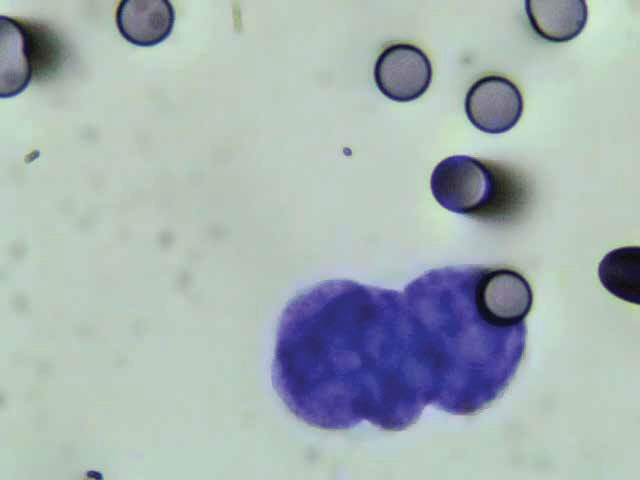
Studies found that different cancer types responded to cancer or pathogen specific frequencies.
In this study, we will use an EMF device plus plasma tube, and cancer specific range of frequencies as per EMF/Rife catalogue. For clinical outcomes, typically EMF treatment involves multiple 1-2 hr sessions weekly over a period of 3-6 months.
The objectives of this study are to assess the effect of EMF therapy on:
– CTC count on day 2, at 3 months, and at 6 months
– tumour markers at 3 and 6 months
– cancer status and tumour size at 6 months
– quality of life at 3 and 6 months
Who can participate in this study?
1. 18+ years old, not pregnant, and able to give consent
2. do not have electronic implants, e.g. pacemaker, defibrillator, cochlear implant, ring-shaped metals in the body
3. have been diagnosed with cancer, and have a doctor’s referral to the study (CTC test request form)
4. not planning to start any new treatments during the study period (3-6 months) – ongoing treatments are ok
5. able to come to the clinic for weekly/twice weekly visits for ~3 months
6. willing to pay for CTC blood tests and EMF treatment sessions (please contact us for details)
Note: If you have chronic fatigue, EMF therapy might also be useful for you (contact us for details).
How will this project be conducted?
You will need a referral from your doctor to participate in the study.
Consenting patients will be treated with EMF therapy for 60-120 min whilst seated.
Participants will have 2×10 ml of blood taken for CTC testing at four time points, on day 1, day 2, at 3 months and at 6 months.
Contact for more information:
research@niim.com.au
